
Glove Box Work Station
This is a hermetically sealed enclosure built specially to perform sensitive reactions under inert gas atmosphere. In general, nitrogen or argon gas cylinders are used to maintain the inert atmosphere inside the glove box. Air and moisture sensitive chemicals are stored and/or handled inside this box. It works under extrermely air and moisture free condition, where the levels of O2, H2O are strictly maintained at 0.1 ppm. One ppm of oxygen means one milligram of oxygen in one liter volume. This box functions with the help of molecular sieves (absorbs moisture) and activated copper catalyst (kills oxygen) which gives close to oxygen and moisture free environment inside.

Solvent Purification System (SPS)
Commercially supplied organic solvents are generally stored under aerobic condition. Additionally, they contain other chemical impurities. That is why these solvents can not be directly utilised for air and moisture sensitive chemical reactions. The Solvent Purification System contains several columns of silica and activated catalyst where H2O and O2, and other chemical impurities are destroyed supplying air and moisture free high purity organic solvents (5 to 15 ppm). Solvents are collected from the outlet under high flow of nitrogen or argon gas and utilised for the subsequent chemical reactions in the chemical laboratory.
The upper part of the system contains the purification units and the bottom part has the solvent storage containers. SPS provides the safe and easy and faster way to convert HPLC grade solvents to the ultra pure solvents.
Note: The purity of SPS solvent is not sufficient to perform highly sensitive chemical reactions involving radicals etc. This requires further purification of the solvent.
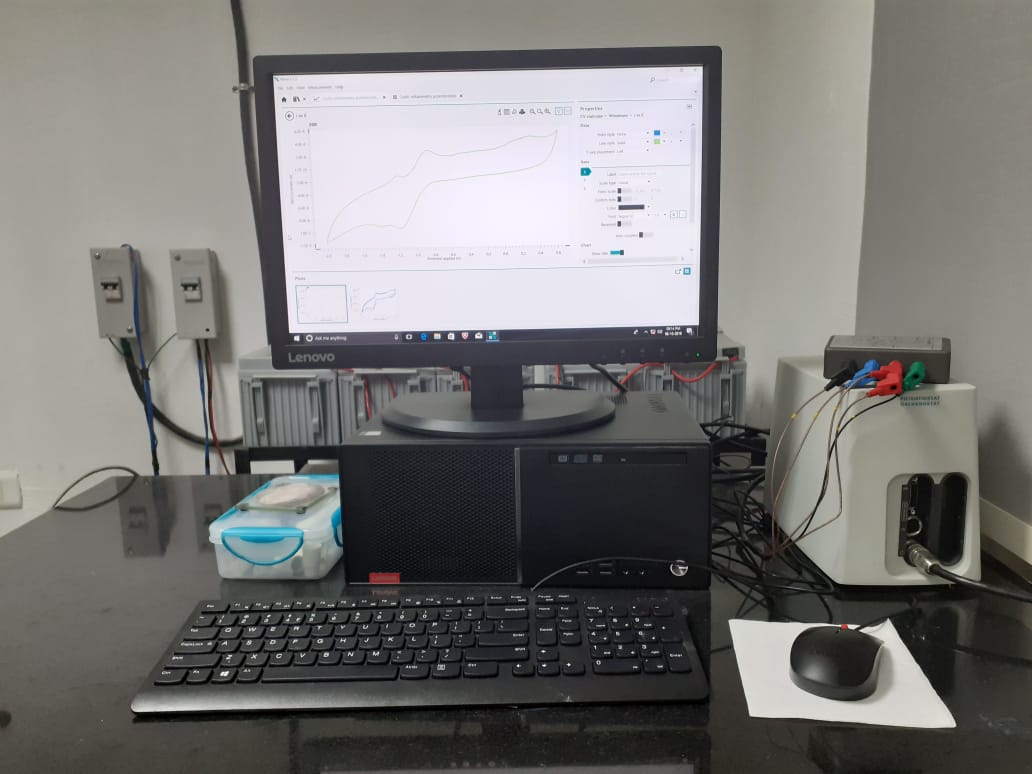
Potentiostat for CV
Any compound (Inorganic, organic or hybrid) can have one or multiple low lying orbitals where electron will be resting or these orbitals remain vacant. The former will try to leave the electron in solution to become cation in solution under applied electrochemical potential. The latter can take one electron to become anion in solution. A solution conatining electrolyte is given electrochemical potential difference between the two electrodes the neutral compound can form a cation or anion. Based on its charge this species can travel between the electrodes by changing the electromagnetic potential at the electrodes. In this way redox property of a species can be studied in solution. By utilising this electrochemical behaviour of a species, solution chemistry of chemicals can be exploited by altering the chemical ingredients of a chemical reaction.

Rotary Evaporator
For synthesis and purification of organic compounds via column chromatography this instrument is mandatory. Organic solvent containing other organic molecules can be separated. Left side glassy part is connected to a vacuum pump and right side solvent containing organic compounds has been placed in a warm liquid bath. During the separation process the round bottom flask conatining the solution is rotated to release the excess vaour pressure inside the solvent. At the end of the separation organic solvent is collected in the left side flask and organic compound remains in the round bottom flak on the right side.

GC-MS
Gas Chromatography - Mass spectrometer (GC-MS). This instrument is run under high purity He, N2 or H2 gas. This is similar to a mass spectrometer. This instrument is loaded with solution conatining ppm level of concentration. The chemicals in the solutions can have different components of different mass. They are ioinsed in the gas phase and they are detected. So different fragnments containing different mass can be detected and based on the mass we can determine/guess the exact chemical formula of the corresponding neutral molecule.