16. Sudipta Roy, Kartik Chandra Mondal, Herbert W. Roesky. Cyclic Alkyl(amino) Carbene Stabilized Low Coordinate Metal Complexes of Enduring Nature.
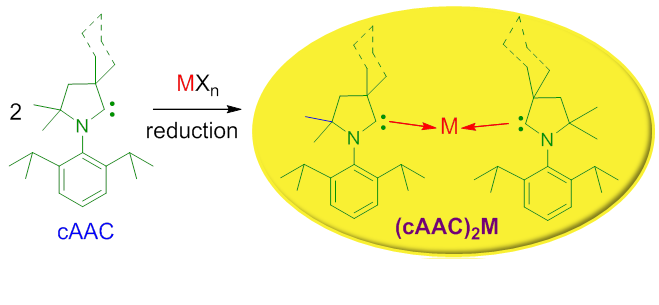
Abstract: N-heterocyclic carbenes (NHCs) are known to stabilize some metal atoms in different oxidation states mostly by their strong σ-donation. After the successful syntheses of cyclic alkyl(amino) carbenes (cAACs), they have been proven to be much more effective in stabilizing electron rich species. In cAAC, one of the σ-withdrawing and π-donating nitrogen atoms of NHC is replaced by a σ-donating quaternary carbon atom leading to a lower lying LUMO. This makes the acceptance of π-back-donation from the element bound to carbene to the carbon atom of cAAC energetically more advantageous. Further evidences suggest that the carbene carbon of cAAC can utilize the lone pair of electrons present on the adjacent nitrogen in a more controlled way depending upon the accumulation of electron density on the metalt bound to it. It has been found that cAACs can be utilized as excellent ligands for the stabilization of complex with three coordinate MI-Cl [(cAAC)2MI-Cl; M = Fe, Co, Cr]. Complex (cAAC)2MIICl2 [M = Fe, Co, Cr] was prepared by reacting anhydrous M(II)Cl2 with two equiv of cAAC and then (cAAC)2MIICl2 was treated with one equiv of KC8 (reducing agent) to obtain (cAAC)2MI-Cl. The corresponding cation (cAAC)2M+ was isolated when (cAAC)2MI-Cl is reacted with the sodium/lithium-tetraarylborate in toluene or fluorobenzene. The CV of (cAAC)2M+ [M = Co, Fe] cations suggest that it can reversibly undergo one electron reduction. The cations of Co and Fe were reduced with Na(Hg) or KC8 respectively. (cAAC)2CoICl can be directly reduced to (cAAC)2Co0 when reacted with one equiv of KC8. Analogous (cAAC·)2ZnII and (cAAC)2Mn complexes are prepared by reduction of (cAAC)MCl2 [M = Zn, Mn] with two equiv of KC8 in the presence of one equiv of cAAC. The square planar (cAAC)2NiCl2 complex was directly reduced by two equiv of LiN(iPr2)/KC8 to (cAAC)2Ni0 complex. The (cAAC)2Pd0 and (cAAC)2Pt0 complexes are prepared by substituting all four triphenylphosphines of (Ph3P)4M0 [M = Pd, Pt] by two cAACs. Cation (cAAC)2M+ [M = Cu, Au] were reduced with sodium/potassium to obtain their neutral analogues (cAAC)2Cu/(cAAC)2Au. Two coordinate Zn/Mn/Cu/Au are stabilized by two neutral carbene ligands possessing radical electrons on carbene carbon atoms while analogous complexes of Co/Fe/Ni/Pd/Pt contains metal in the oxidation state zero. The ground electronic structures of (cAAC)2M are thoroughly studied by theoretical calculations. In this account, we summarize our developments in stabilizing metal complexes with low coordinate metal atoms in two, one, and most significantly in their zero oxidation states by utilizing cAACs as the ligands.