14. Kartik Chandra Mondal, Sudipta Roy, Bholanath Maity, Debasis Koley, Herbert W. Roesky. Estimation of σ-Donation and π-Backdonation of Cyclic Alkyl(amino) carbene Containing Compounds.
Abstract:
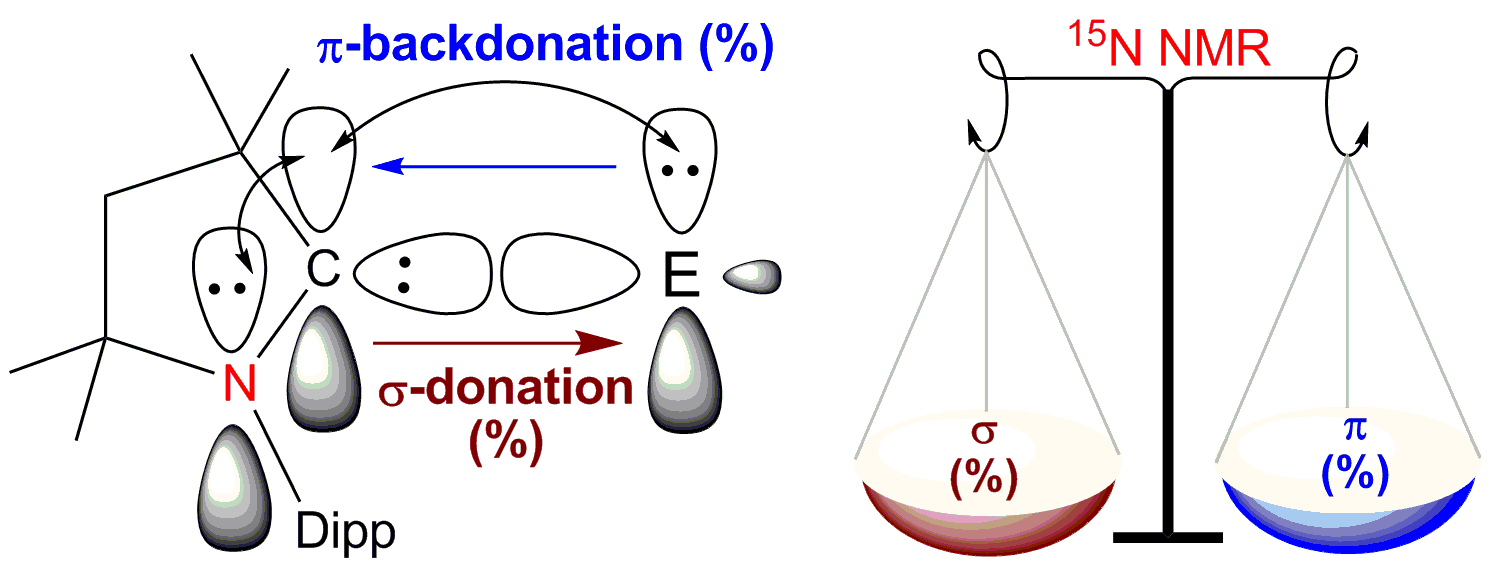
A large number of previously reported experimental results show that the cyclic alkyl(amino) carbenes (cAACs) are better π-accepting than NHCs. Additionally cAACs form different type of bonds (coordinate σ-bond, donor-acceptor partial double bond, and covalent electron sharing single bond). Herein, we present a general method for the reliable estimation of the extent of π-backdonation (CcAAC←E) of the bonded element (E) to the carbene carbon atom and CcAAC→E σ-donation. The CcAAC←E π-backdonation has significant effect on the electronic environments of 15N nucleus. Hence the estimation of the π-backdonation has been achieved by recording the chemical shift values of 15N nuclei from two dimensional heteronuclear multiple-bond correlation spectroscopy (15N-HMBC). The chemical shift values of 15N nuclei of several cAAC-containing compounds/complexes are recorded. The 15N NMR chemical shift values are in the range from -130 ppm to -315 ppm. When the cAAC forms a coordinate σ-bond (CcAAC→E), the chemical shift values of 15N nuclei are around -160 ppm. When cAAC is bonded to cationic species, the numerical chemical shift value of 15N nucleus is downfield shifted (-130 to -148 ppm). The numerical values of 15N nuclei fall in the range from -170 to -200 ppm when the σ-donation (CcAAC→E) of cAAC is stronger than CcAAC←E π-backacceptance. When π-backacceptance of cAAC is higher than σ-donation, the chemical shift values of 15N nuclei are observed below -220 ppm. Electron density and charge transfer between CcAAC and E are quantified using NBO (natural bonding orbital) analysis and CDA (charge decomposition analysis) techniques. The experimental results have been correlated with the theoretical calculations. They are in good agreement.