12. Sudipta Roy, Kartik Chandra Mondal, Totan Mondal, Debasis Koley, Birger Dittrich, Herbert W. Roesky. Monomeric siliconthiodichloride trapped by a Lewis base.
Abstract:
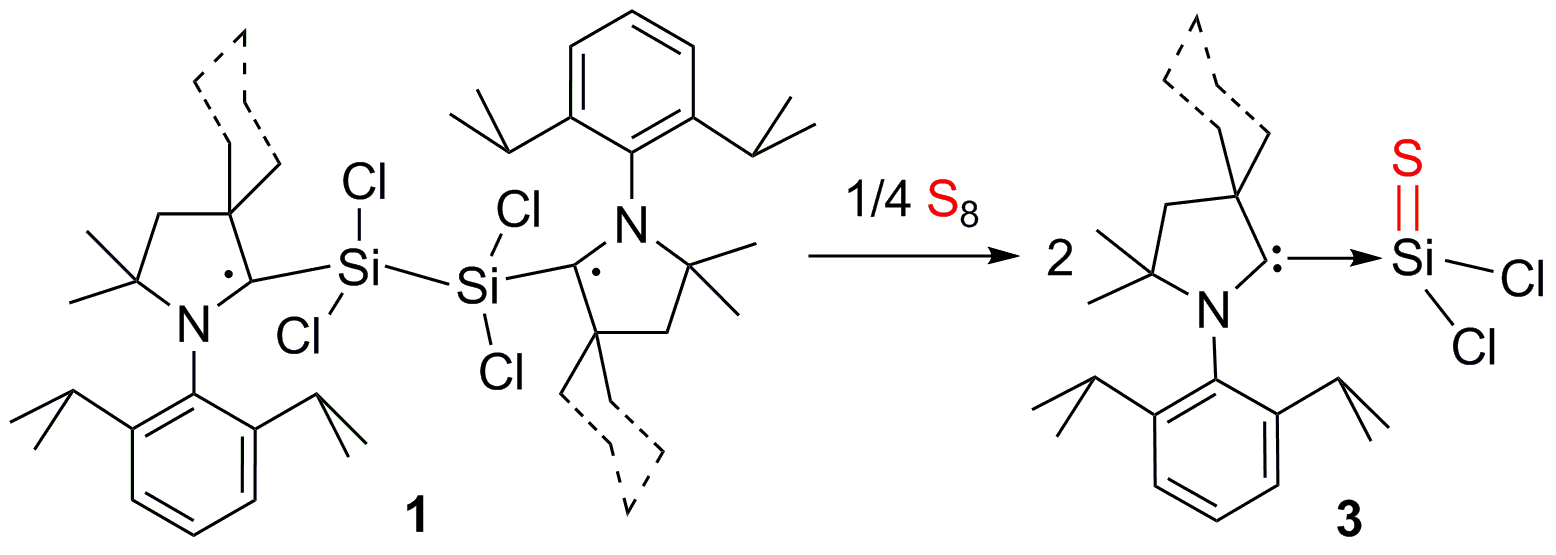
Thiophosgene (CSCl2), a chemical reagent used in numerous organic syntheses, exists in the monomeric form while its heavier silicon analogue [siliconthiodichloride (SiSCl2)] is isolated so far as a dimer at room temperature and tetramer at 180 °C. Herein, we report on the first synthesis, isolation, and characterization of cyclic alkyl(amino) carbene (cAAC) stabilized siliconthiodichloride (cAAC)SiSCl2 (3) in the neutral monomeric form. 3 is synthesized via reaction of (cAAC·)2Si2Cl4 (1) or (cAAC)2Si2Cl2 (2) with S8 in the temperature range of -78 to 20 °C. An NHC [NHC = N-heterocyclic carbene] analogue of 3 is not isolated when (NHC)SiCl2 is reacted with S8. The bright yellow colored compound 3 is soluble in polar organic solvents. It is stable at room temperature for a month under an inert atmosphere. 3 decomposes above 160 °C. The monomeric molecular structure of 3 has been unambiguously confirmed by X-ray single crystal diffraction. 3 is also characterized by NMR, UV-vis, and IR spectroscopy. The bonding and electron density distributions of 3 have been further studied by theoretical calculations.